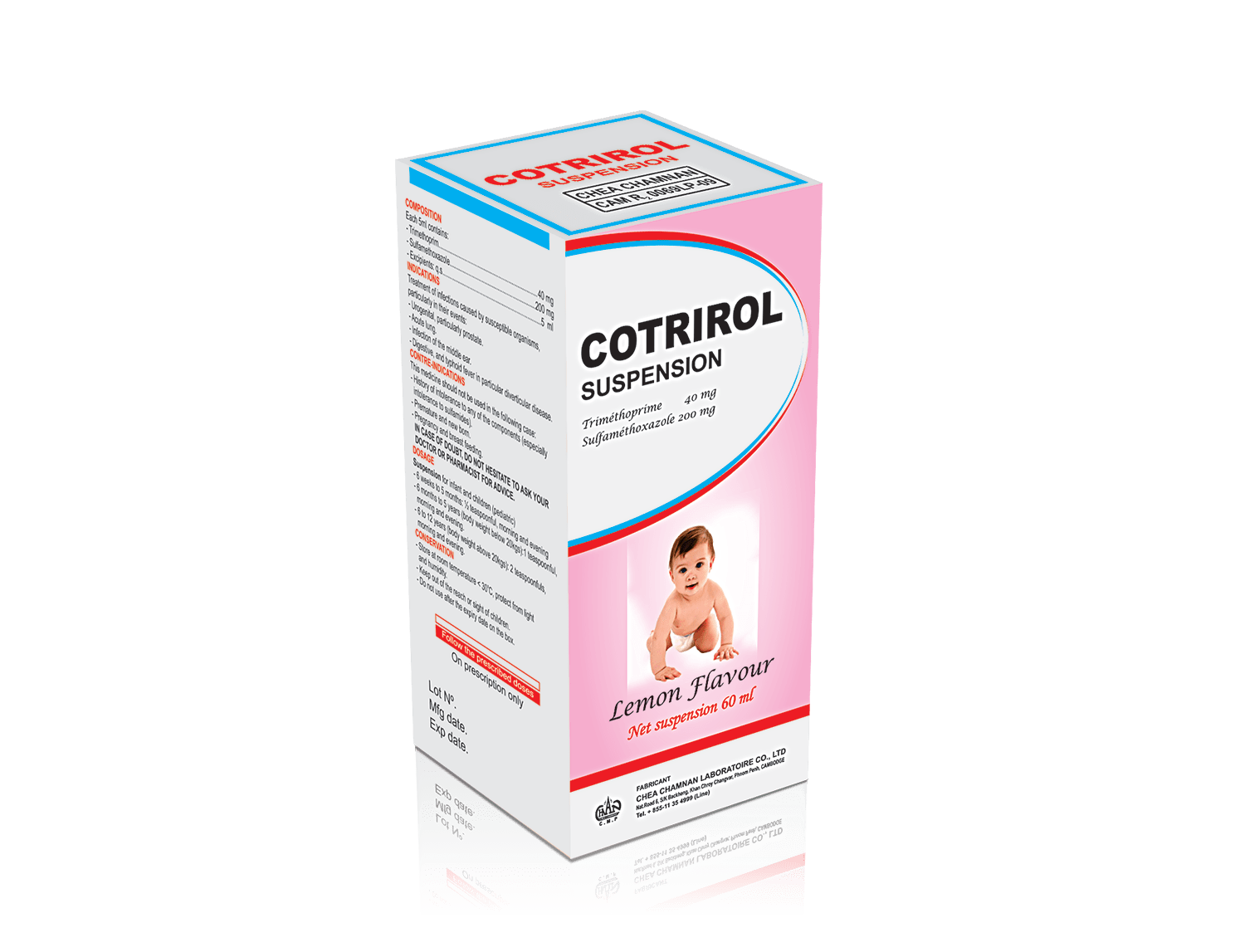
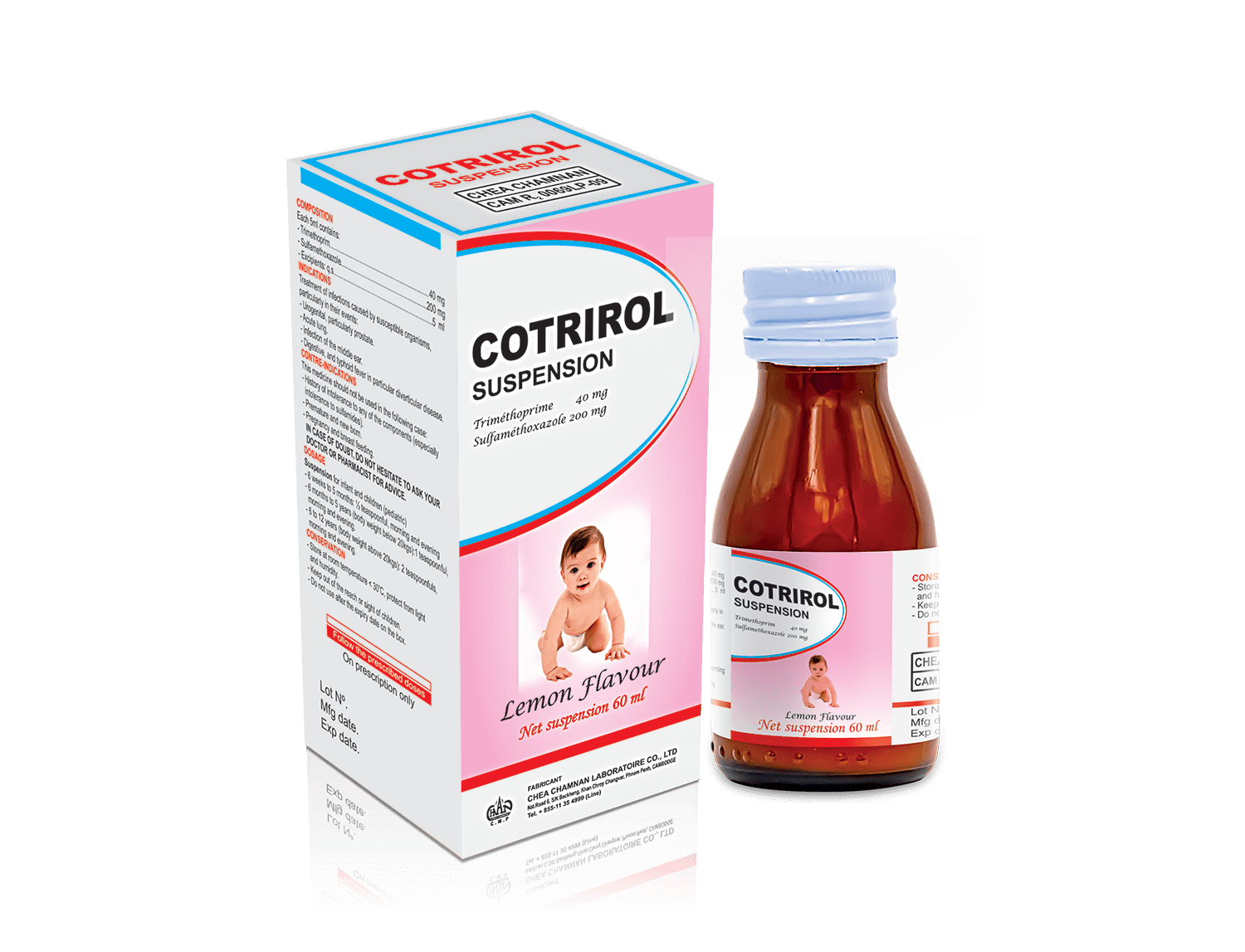
COTRIROL Suspension
COMPOSITION
Each 5ml contain :
Sulfamethoxazole : ………………………………………………. 200 mg
Trimethoprim : ……………………………………………………….40 mg
Excipients : q.s …………………………………………………………..5ml
THERPEUTIC PROPERTIES
This medication is a combination of two antibiotics: sulfamethoxazole, from the sulfonamide class, and trimethoprim, from the diaminopyrimidine class.
INDICATIONS
They are indicated for the following conditions:
- Urogenital, particularly prostate-related issues.
- Acute bronchopulmonary infections.
- Middle ear infections.
- Digestive infections, particularly typhoid fever.
CONTRAINDICATIONS
This medication should not be used in the following cases:
- A history of intolerance to any of the components (particularly sulfonamides).
- Premature infants or newborns.
IN CASE OF DOUBT, IT IS NECESSARY TO ASK
FOR THE ADVICE OF YOUR DOCTOR OR PHARMACIST.
WARNING AND PRECAUTION
Hematological monitoring is recommended for prolonged treatment.
SIDE EFFECTS
- Digestive disorders: nausea, stomach pain.
- Skin manifestations: rash, urticaria. A few cases of epidermal necrolysis have been reported.
- Cases of renal function impairment have been reported
DOSAGE AND METHOD OF ADMINISTRATION
Suspension for infants and children (pediatric)
- From 6 weeks to 5 months: ½ teaspoon in the morning and evening.
- From 6 months to 5 years (body weight less than 20 kg): 1 teaspoon in the morning and evening.
- From 6 to 12 years (body weight greater than 20 kg): 2 teaspoons in the morning and evening.
STORAGE
- Store below 30oC, avoid light and humidity
- Keep out of reach and sight of children.
- Do not use after expiry date.


Reviews
There are no reviews yet.