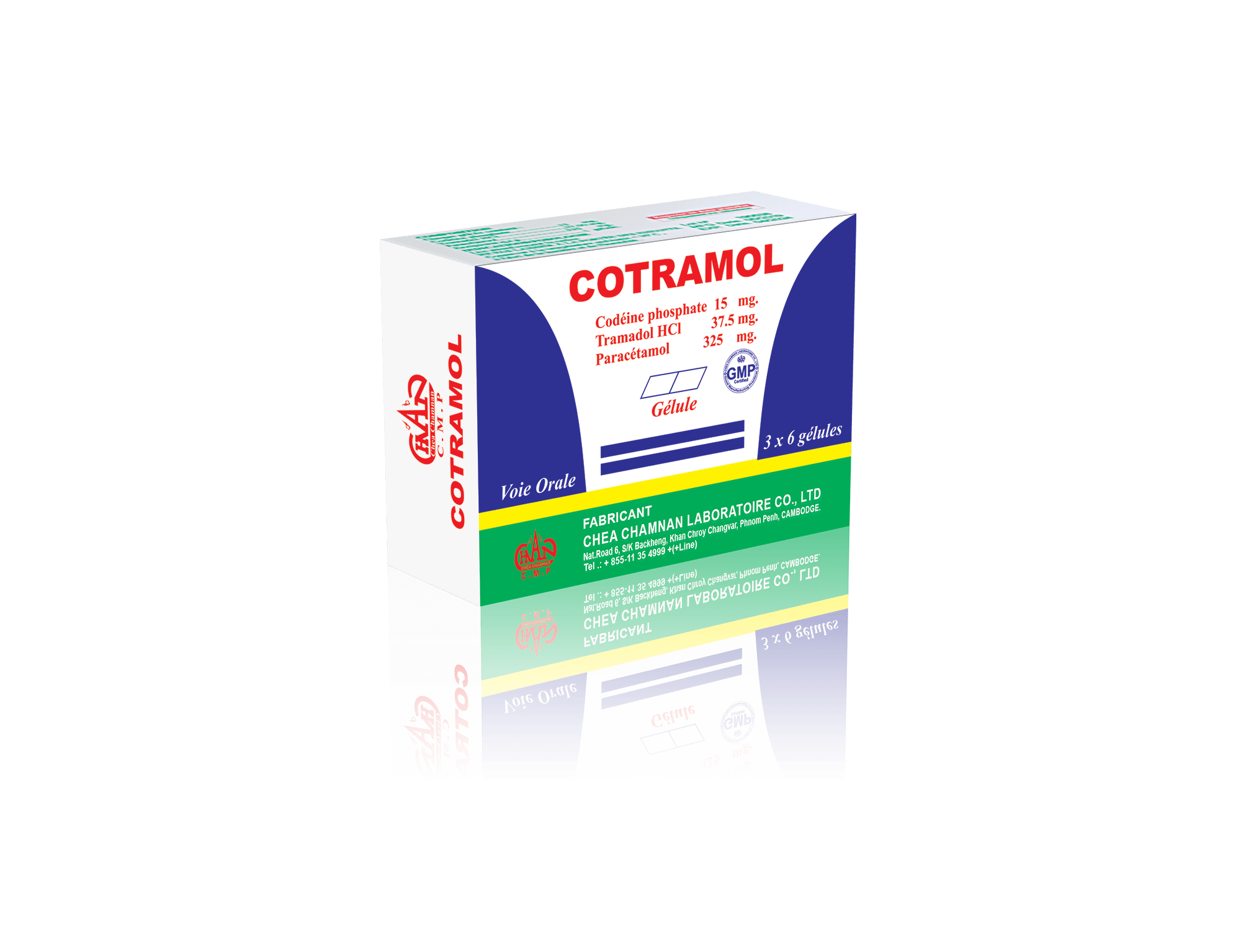
COTRAMOL
COMPOSITION:
Each capsule contains:
Paracetamol……………………………………………………….: 325 mg
Codeine phosphate ………………………………………………………………..: 15 mg
Tramadol HCl……………………………………………………:37.5 mg
Excipients q.s …………………………………………………………………: One capsule
THERPEUTIC PROPERTIES
Central and peripheral analgesics
INDICATIONS :
Symptomatic treatment of moderate to severe pain.
CONTRAINDICATIONS :
- Hypersensitivity to any of the components of the medication.
- Severe hepatic insufficiency.
- Asthma.
- Respiratory failure.
- Children under 15 years of age.
- Pregnant women (unless otherwise advised by a doctor): see Pregnancy/Breastfeeding section.
IN CASE OF DOUBT, IT IS NECESSARY TO ASK
FOR THE ADVICE OF YOUR DOCTOR OR PHARMACIST.
PRECAUTION AND WARNING :
Precautions:
- In chronic respiratory insufficiency, codeine may cause bronchoconstriction and respiratory depression.
- There is a risk of drowsiness for vehicle drivers and machine users due to the presence of codeine.
Warning:
- Prolonged use of high doses of codeine may lead to a state of dependence.
SIDE EFFECTS:
- Seizures
- Nausea and vomiting
- Drowsiness, headaches, dizziness, sweating, and feeling unwell
- Dry mouth
- Constipation with prolonged use
DOSAGE:
Oral route
- Reserved for adults and children over 15 years old: 1 to 2 capsules depending on the severity of the pain, 1 to 3 times a day, with a large glass of water. Doses should be spaced at least 4 hours apart.
- For individuals aged 75 and older, it is recommended to increase the interval between doses (to every 9 hours).
- In cases of severe renal insufficiency (creatinine clearance less than 10 ml/min), the interval between doses should be at least 8 hours.
CONSERVATION :
- Store below 30oC, avoid light and humidity
- Keep out of reach and sight of children.
- Do not use after expiry date.



Reviews
There are no reviews yet.